Dandruff and seborrheic dermatitis
Dermatology No 1 2007 SKIN DISEASES: DISEASES OF SEBACEOUS GLANDS
А.G. Hajigoroyeva
Public Enterprise Central Research Institute of Dermatology and Venereology, Moscow
Physiology of the scalp skin
The skin of the scalp is characterized by a large number of large sebaceous glands. This is due to the considerable amount of hair in this area, since the hair follicle is a single pilosebaceous unit. The number of units can reach 460/cm2. The size of sebaceous glands has variations associated with hair: large sebaceous glands of the 1st order are connected with long hair, smaller glands of the second order are connected with lanugo hairs. In very small quantities, only 2–4 cm2, there are small monocotyledonous glands of the third order [1].
In addition to the presence of long terminal hair and the high level of production of sebum, the scalp is characterized by enhanced desquamation of keratinized non-nuclear cells from its surface. This continuous natural physiological process is necessary to maintain the tissue homeostasis of the epidermis and provide a number of protective functions of the skin.
The structural integrity of the skin, immune and biochemical means of protection play an important role in the formation of barrier and protective functions of the stratum corneum of the epidermis. Barrier skin functions are provided by acidic and water-lipid mantle, epidermis with stratum corneum, intercellular cytotoxic cement, barrier area between corneocytes and granular cells, skin-associated lymphoid tissue, intraepidermal phagocytic system, etc.
The skin surface has the property of self-sterilization, provided by the water lipid mantle and the special skin microbiocenosis, formed as a result of the symbiosis of numerous microorganisms inhabiting the surface of the skin. The composition of the microflora of the skin of the head and hair is different. Saprophytic and pathogenic microorganisms: bacteria, filamentous fungi, lipophilic yeasts are found in scrapes from the skin and scratches from hair. Saprophytes refer to resident inhabitants of the skin and most of them are considered as normal physiological skin microflora. On a healthy skin, normal microflora is relatively stable and shows colonization resistance. In flakes, molds are often found that correspond to the species composition in the environment and usually have no clinical significance. Pathogenic bacteria and fungi can cause such known diseases of the skin of the head and hair as microsporia, trichophytia, pyoderma.
During the violation of the integrity of the barrier and protective functions of the epidermis and its stratum corneum, there is a violation in the system of limiting the penetration of microorganisms and enhancement of their pathogenic effect, which may lead to disturbance of desquamation and erythro-squamous lesions of the scalp.
Usually skin cells are renewed without attracting attention. When there is no dandruff, the nail drawn on a healthy scalp that is not affected by the disease, will scrape only a small amount of lightly oily white flakes, indicating a normal skin renewal. Migration of skin cells from the lower layers of the epidermis to the upper stratum corneum takes 28 days. The cells are exfoliated with imperceptible to eyes flakes.
Enhanced desquamation
Almost every person encounters at least once in his/her life with the easiest manifestation of increased desquamation of the cells of the scalp – dandruff. Statistically, dandruff is more common in Chinese Americans, observed in 83% of the adult population [2]. The sign of dandruff is desquamation of the stratum corneum in the form of greasy white flakes, which are especially noticeable on dark clothes, in hair and on the scalp. Too large scales indicate excessive production of sebum by the skin of the head and too rapid cell renewal. While flakes make dandruff noticeable, the patient may suffer from itching and squeezing the skin without visible signs of hyperproliferation of the cells. Migration of the cell in case of dandruff lasts for a short period: 7–21 days, the cells fall in flakes of 100–1000 cells.
Aetiopathogenesis
The predisposing factors of the appearance of dandruff is the excessive production of sebum due to the functional activity of the sebaceous glands, hormonal changes, or a diet. Often, the first manifestations of dandruff occur during puberty, when, under the influence of androgens, sebaceous glands begin to work in full force. A significant increase in the production of sebum leads to increase in colonies of lipophilic microorganisms.
The assumption that the cause of dandruff can be Malassezia fungi, was firstly expressed by the French microbiologist Louis Charles Malasses (1842–1909) about a hundred years ago, when he first discovered a yeast-like substance in the scales of a patient with seborrheic dermatitis. This was an opening in mycology, but the picture was far from clear.
In 1984, S. Schuster proved that the yeast fungus Pityrosporum ovale is the main causative agent of the pathological process, because of which dandruff is produced [3]. Once the lipophilic nature of these yeasts was recognized and the growing of their cultures became possible, various researchers observed spontaneous transitions from one morphological type to another, leading to the conclusion that Pityrosporum ovale, Pityrosporum orbiculare and Malassezia furfur are only variants of one and of the same species [4].
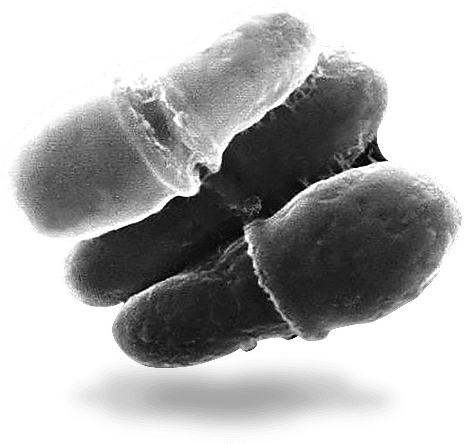
When Pityrosporum was re-classified in seven Malassezia genus species [5–8] in 1996, Malassezia furfur was recognized as the microorganism responsible for the formation of dandruff and seborrheic dermatitis. At least 90% of the population have these yeast fungi in the permanent or temporary microflora of the skin. Fungi of the genus Malassezia belong to imperfect yeast-like fungi, basidiomycetes. Fungi are localized in the middle and surface parts of the stratum corneum, inside and between horny scales, and in the hair follicles [9, 10]. The fungi are lipophilic, some are even lipodependent. This explains the habitat of microorganisms: skin areas characterized by increased activity of the sebaceous glands: chest, back, scalp [11]. The main source of lipids for Malassezia is the saturated fatty acids produced by sebaceous glands: triglycerides of oleic acids with small concentrations of stearic and palmitic acids or lipids obtained after the disintegration of keratinized cells, mainly cholesterol and cholesterol esters [12].
For a long time, the microorganism Malassezia furfur was considered to be the cause of seborrheic dermatitis and dandruff, which is the most easily cultivated variety of the fungus and is widespread everywhere. However, the specialists were bewildered by inconsistency: the lesions by M. furfur of other parts of the body look quite different than the scalp peeling in case of dandruff or seborrheic dermatitis. Conducting genetic studies has finally allowed to identify the exact component of microflora, first discovered by the researcher Malasses a century ago. Using genomic methodology, P&G Beauty researchers increased fungal ribosome clusters of ITS I and ITS II genes from each DNA sample and analysed the number of their fragments in order to differentiate each Malassezia species. In the analyses from the surface of the scalp, the fungus Malassezia furfur was not detected [13]. In addition, the prevalence of two previously unknown fungi was found: M. restricta and M. globosa, which excluded M. furfur from the list of “suspects”. Yeasts, inhabiting the human scalp, were much more complex than originally thought. Mistakes in the diagnosis of M. furfur as the true cause of dandruff and seborrheic dermatitis are explained by the specifics of their previous definition. The microflora was grown on Petri dishes in which M. furfur was easily cultivated, rapidly multiplied and completely eclipsed the signs of the presence of other fungi, while M. globosa was poorly grown with this method of cultivation, multiplying in a much more specific environment of the scalp. Therefore, the presence of species M. restricta and M. globosa were detected only as a result of gene research.
To date, more than 9 species of Malassezia fungi are known. More accurate identification, carried out by the researchers, determined the mode of action of the fungus and the mechanism that causes increased peeling of the scalp. Despite the fact that the number of M. restricta on the surface of the skin of the scalp exceeds the number of M. globosa, the latter have higher lipase activity, which is the main cause of irritation and dermatitis in seborrheic dermatitis. Consuming sebum triglycerides, M. globosa secretes unsaturated fatty acids, components of which cause skin irritation [14]. Irritation leads to inflammation and flaking, which weakens the barrier function of the skin. Oleic acid, which is part of the sebum, is very irritating to the skin itself. It is proved that under appropriate conditions, oleic acid can cause peeling, as in dandruff. In the experiment, oleic acid was applied to the scalp of patients prone to dandruff, and there was increased sebaceous excretion and excessive desquamation of the stratum corneum, similar to that which occurs during dandruff formation [15, 16].
Anatomical and physiological features of the scalp with large quantity of hair and sebaceous glands, high levels of sebaceous excretions and speed of desquamation, with availability of scales, secretion of sebaceous and sweat glands on the skin and hair, heat and humidity create favourable conditions for permanent and temporary resident microorganisms. Scratches and scalp excoriation in case of itchy dermatoses cause damage to the epidermal barriers, scaly skin formation and inflammation.
Nosological identification of lesions on the skin of the scalp in practical work is complicated by the fact that not all pathological conditions and nosological units are sufficiently separated in the scientific and reference literature due to the lack of fundamental data and different opinions about their pathogenesis. Until recently, it also concerned dandruff (syn. seborrhea furfuracea of scalp) and seborrheic dermatitis. However, modern knowledge of the nature of dandruff and seborrheic dermatitis suggests that dandruff is a mild clinical form of seborrheic dermatitis and can be transformed into it under a combination of three conditions:
- hypersensitivity of the skin to the formation of inflammatory reactions to the action of oleic acid as a result of innate tendency;
- the presence of sebum as a nutrient medium for fungi;
- the presence of M. globosa in the composition of the microflora of the scalp.
In adults with a healthy immune system, the incidence of seborrheic dermatitis ranges from 1 to 3% [17], in patients with HIV this rate is higher and occurs from 30 to 83% [18, 19]. Often, seborrheic dermatitis occurs in patients with psoriasis, Parkinson’s disease, depressive conditions, and those treated with ultraviolet A with psoralen (PUVA) [20–23].
Although currently the role of the yeast of the Malassezia species is the principal in the formation of seborrheic dermatitis, the exact etiology of the disease is debated. Some authors suggest an abnormal immune response to Malassezia, despite the vagueness of the manifestation of this process. Some researchers confirm this theory by detecting an increase in the level of IgG antibodies to Malassezia in patients with seborrheic dermatitis [24, 25]. In other studies, no differences were found in the amount of antibodies in patients with seborrheic dermatitis compared with healthy ones [26]. Recently it was found that the reaction to Malassezia yeast is more characteristic of irritation and inflammation [27]. Subsequent studies have shown that the reduction of Malassezia yeast on the skin as a result of taking oral and local antifungal drugs reduces the clinical manifestations of seborrheic dermatitis [28].
The disruption of normal microbial biocenosis of the scalp skin can result from the pathology of the organs of the gastrointestinal tract: fermentopathy, dysbacteriosis, exacerbation of chronic diseases of the gastrointestinal tract. Therefore, a thorough clinical and laboratory research, including the determination of the main biochemical parameters, blood sugar, the detection of intestinal dysbiosis, the study of endocrine status, is necessary in a comprehensive clinical and laboratory diagnosis of seborrheic dermatitis.
Therapeutic Management of Dandruff
The appearance of dandruff under certain physiological conditions (pre- and puberty) is a normal physiological state and does not require the use of drug antimicrobial agents in order to eliminate the cause of dandruff. Timely and adequate hygienic hair care will allow you to feel comfortable in conditions of increased dandruff production as a result of increased activity of sebaceous glands, caused by the physiological increase in androgens during adolescence and youth. In cases where dandruff is the cause of discomfort, you should use a shampoo with an active ingredient against dandruff, which meets the safety requirements when used at home, maintaining its properties in the presence of surfactants and other ingredients of the shampoo. These conditions are met by shampoos containing an antimicrobial agent in the form of zinc pyrithione. The particles of zinc pyrithione, getting on the scalp, slowly dissolve in the secret of sebaceous and sweat glands, forming a “zone of inhibition” of Malassezia. These reservoirs of active antifungal substances provide a continuation of the effective fight against the cause of dandruff after washing off the shampoo in the intervals between its use. The cause of excessive peeling of the scalp can be itching caused by the irritating effect of sebum and the waste products of bacteria that inhabit the surface of the skin. These symptoms may occur as a result of inadequately selected hygiene products for hair care such as an unsuitable type of hair shampoo. Back in the 50s of the last century, Leningrad and other domestic scientists carried out a series of works that demonstrated the sterilizing property of the skin and found out the influence of exogenous and endogenous factors on this function. It was shown that treating the skin with a fat solvent, just like cooling, anesthesia with novocaine, reduces the bactericidal action of the skin. In this regard, shampoo for daily use should have a mild washing base and not lead to drying and defatting the surface of the scalp.
Therapeutic Management of Seborrheic Dermatitis
In the systemic treatment of seborrheic dermatitis, the focus is on enzyme preparations that improve the digestive process (Essentiale, Festal, Carsil), essential amino acids with lipotropic effect, contributing to the utilization of fat accumulations by converting them into energy. In particular, the appointment of methionine helps to normalize the synthesis of phospholipids from fats and reduce the deposition of neutral fat in the liver, promotes the synthesis of adrenaline, creatinine, activates the action of a number of hormones, enzymes, vitamin B12, ascorbic and folic acids. The use of riboflavin regulates redox processes due to the participation of the vitamin in protein, fat and carbohydrate metabolism. In the case of dysbacteriosis, it is necessary to conduct a complex of therapeutic measures that normalize the intestinal flora.
Topical treatment of seborrheic dermatitis is aimed at relieving clinical symptoms of the disease with antimicrobial agents. In this regard, medical management includes the use of etiotropic and pathogenetic agents that help reduce inflammation, itching, regulate sebaceous excretions, have fungicidal and fungistatic effect, and normalize desquamation.
Traditionally, seborrheic dermatitis was treated with topical steroids, leading to a reduction in redness and inflammation; however, atrophy and telangiectasia were common undesirable side effects after prolonged use. New scientific discoveries, which proved Malassezia’s involvement in the development of seborrheic dermatitis, changed the treatment strategy and shifted the emphasis from corticosteroids to antifungal agents. At the same time, the incidence of adverse events has decreased [29]. Clinical experience in the treatment of dermatitis includes the use of various local antifungal drugs that are potentially capable of treating seborrheic dermatitis on the head and other anatomical areas, including eyebrows, middle part of the chest, and ears. The list of agents used to treat seborrheic dermatitis includes hydroxypyridone, azoles, allylamines, topical immunomodulators and various nonspecific agents [30–34].
Among many antifungal drugs intended for topical use, preference should be given to those of them, which, firstly, can accumulate in those layers of the skin where the fungal process develops, secondly, do not penetrate into those layers where the activity of fungi is impossible and, thirdly, to have an anti-inflammatory and kerato-regulating effect.
In an effective fight against the manifestations of dandruff and more severe forms of seborrheic dermatitis with localization in the area of the scalp, an important role belongs to modern cosmetics – shampoos and lotions. The technologies underlying the new generation of hygienic and therapeutic agents for dandruff make it possible to achieve a high therapeutic effect, while having a wide range of safety, aesthetic advantages and being comfortable to use.
In general, it can be said that the identification of M. globosa improved the understanding of the role of fungi in the development of dermatitis of the skin of the body and reduced the long-term use of corticosteroids. However, in some cases, seborrheic dermatitis may require in addition to topical and oral use of antifungal agents, the use of steroids.
Clinical trials of anti-dandruff shampoos with a monograph antimicrobial ingredient
Zinc pyrithione is one of the most common fungicidal agents used to fight dandruff in various cosmetic and medical preparations.
In one study, a shampoo containing 1% zinc pyrithione was used in experiments on one side of the head in 32 people with dandruff, the other side was washed with a usual shampoo. In four groups of participants, 8 people in each group, there was used a shampoo for 1, 3, 6 and 9 times (2 times a week) for washing the heads. Evaluation of the results was carried out in each group on both sides of the head 4 days after the last washing of the head. A comparison of the mean score showed a progressive decrease in dandruff on the side of the head, which was washed using a shampoo containing zinc pyrithione. Significant differences (p <0.05) of shampoos were observed after the third shampooing, significant differences (p <0.01) – after 6 and 9 applications of shampoos (Wilcoxon test) [35].
G.A. Dmitriev et al. studied the fungicidal activity of a shampoo containing 1% zinc pyrithionate, in vitro. The results showed that the minimum inhibitory concentration of the shampoo with respect to Malassezia was 250 μg/ml, or 0.025%. It was found that the suppression of the growth of the culture of fungi occurred within the first hour, and the absolute absence of growth was noted after 48 hours with an initial titre of 60,000 CFU/ml. Electron microscopic studies have shown morphological changes in yeast cells under the influence of shampoo at a concentration of 0.025% in the form of detachment of the cell wall and the formation of a fine granular substance on the surface of fungal cells. After 24 hours of exposure to the fungicidal dose of shampoo, the fungus cells lost their oval shape, there were “faults” between the parent and daughter cells, which confirmed the violation of cell division and indicated the Malassezia lysis [36].
Clinical tests show that zinc pyrithione is also able to eliminate superficial skin irritation.
Ketoconazole is an antifungal drug for external use, a derivative of imidazoldioxolane. It has fungicidal and fungistatic effect. The mechanism of action is to inhibit the biosynthesis of ergosterol and change the lipid composition of the membrane of fungi. Along with dermatophytes, the drug is active against yeast and yeast-like fungi Candida, Pityrosporum (Malassezia) [37]. Ketoconazole may be prescribed for oral administration. In the case of the medicinal form of the drug for external use, ketoconazole should be applied to the scalp for 5–10 minutes. It is not recommended for patients under 12 years old and should not be used on irritated skin. Usually used for short-term therapy, several times a week alternately with non-therapeutic shampoo.
Cyclopirox is a derivative of hydroxypyridone, a synthetic antifungal agent that also has a pronounced anti-inflammatory effect. Unlike other antifungal agents such as azoles, ciclopirox does not affect sterol synthesis. Instead, ciclopirox inhibits the uptake of the necessary compounds by the cell [38]. In high concentrations, ciclopirox acts on the membranes of yeast-like cells, changing cell permeability. In one study, patients with lichen-coloured lichen, the etiology of which is associated with the species of Malassezia furfur, periodically received a solution of 1% cyclopirox. Fifteen days after treatment, the cell wall and the internal structure of the Malassezia yeast were damaged, which was confirmed by studies using an electron microscope [39].
Cyclopirox can act through the internal complexation of polyvalent cations, such as Fe3+. The products of this chelation inhibit the effect of metal-dependent enzymes responsible for the degradation of pyroxides in fungal cells [40, 41].
In vitro, ciclopirox has proven to be a broad-spectrum antifungal agent. Its fungistatic or fungicidal effect extends to a wide range of fungal organisms, including dermatophytes, yeasts, dimorphic fungi, eumycetes and actinomycetes [42], and is also active against many gram-positive and gram-negative bacteria [43]. The dose and duration of treatment depend on the type of pathogen and the dosage form used. When applied topically, ciclopirox quickly penetrates the skin and its appendages. It is known that for pathogenic fungi the inhibitory concentration of the drug in the tissues occurs after 1.5 hours [44]. However, on two main types of fungi that cause the development of seborrheic dermatitis, M. globosa and M. restricta, ciclopiroxolamine has a powerful fungicidal effect after 3 minutes of contact [45].
The anti-inflammatory effect of ciclopirox is found in human polymorphonuclear cells, where ciclopirox inhibits the synthesis of prostaglandins and leukotrienes [25]. Ciclopirox has anti-inflammatory effect due to inhibition of 5-lipoxygenase and cyclooxygenase [28].
Numerous clinical studies conducted in various scientific centres have shown the efficacy and safety of a shampoo containing 1% ciclopirox in the treatment of seborrheic dermatitis on the head [46–48]. In particular, the researchers D. Vardy et al. [34] conducted a randomized, double-bound, placebo-controlled study of the efficacy of 1% ciclopirox shampoo in 102 patients with seborrheic dermatitis on the scalp. All patients were randomized according to the shampoos used: 53 patients used shampoo with 1% ciclopirox, 49 patients used regular shampoo 2 times a week for 4 weeks. The presence and severity of symptoms such as erythema, desquamation, itching, just like the general evolution of dermatosis, were determined at the end of treatment. By the end of treatment, the differences were more pronounced in the group that received 1% shampoo with ciclopirox compared to regular shampoo: 93 and 41%, respectively (p <0.00001).
A safety assessment of ciclopirox shampoo, conducted in several randomized dual related controlled studies, showed that the incidence of side effects on the skin, possibly associated with the shampoo, is the same in both treatment groups with active shampoo containing ciclopirox and those using conventional shampoo [34, 46–48].
In the treatment of seborrheic dermatitis, other medicinal forms of ciclopirox are also active, including creams [49, 50], gels [51], and combinations with other substances in shampoos [52, 53]. A detailed analysis of the clinical efficacy of various dosage forms with ciclopirox, including shampoos containing 1.5% ciclopirox, on the evolution of seborrheic dermatitis is given in the article of Aditya K. Gupta et al. [54].
Other effective therapeutic ingredients active against dandruff caused by the fungus Malassezia are selenium sulphide, climbazole and octopirox.
Selenium sulphide has excellent antifungal properties and slows down the cell renewal cycle due to its antimicrobial and cytostatic effects. The products containing selenium sulphide are generally safe and effective for the treatment of seborrheic dermatitis and dandruff. However, patients with coloured hair should follow the instructions and rinse hair thoroughly in order to wash off all the remnants of shampoo containing selenium disulphide due to possible bleaching of hair.
Climbazole has a fungicidal efficacy equal to that of zinc pyrithione. The determination of the minimum effective concentration of drugs showed that they both suppress the growth of Malassezia fungi in the same concentration, equal to 4%. In contrast, for example, to octopirox, when to achieve efficacy against the Malassezia fungus, comparable to that of zinc pyrithione, the octopirox concentration should be 6 times higher.
Other ingredients of anti-dandruff shampoos: keratolytic and cytostatic agents for topical use
Salicylic acid, which is traditionally used by dermatologists as a keratolytic agent, helps remove dead scales. Although it cleanses the scalp, its use can cause inflammation and pronounced dryness of the skin, since salicylic acid suppresses the secretion of sebaceous and sweat glands. For the treatment of desquamative dermatoses, prescription formulations, in which salicylic acid is combined with an antimicrobial drug, are recommended.
For the treatment of desquamation disorders, tar-based detergents are effective. Tar reduces proliferation of the epidermis and skin infiltrates by reducing the number and size of epidermal cells. As an antifungal agent, tar is less effective. Side effects include increased photosensitivity, and taking into account the applicability in cosmetology, it is necessary to note the characteristic medical smell and the tendency to a slight colouring of hair into orange colour, which limits its use.
In case of localization of the pathological process in the area of the scalp the most convenient form of treatment is the use of a medicinal shampoo, which should solve the following tasks:
1. Prevent the growth of yeast fungi.
2. Stop peeling and eliminate its signs, reduce sebum secretion.
3. Slow down the process of cell renewal.
Neither monographs (tar, salicylic acid, selenium sulphide, zinc pyrithione, sulphur), nor nonmonographs (climbazole, octopyrox, ketoconazole) are able to solve all the problems separately. Therefore, our area of interest is focused on products in which active therapeutic agents are combined to influence the etiological and pathogenetic aspects of seborrheic dermatitis. One of such combined products is Kelual DS shampoo (manufacturer “Pierre Fabre”, “Ducray”, France). The active ingredients in the shampoo include cyclopyroxolamine 1.5%, zinc pyrithione 1% and keluamide 1.5. The combination of two active antifungal agents – zinc pyrithione and ciclopiroxolamine – has a synergistic fungicidal effect on Malassezia fungi and relieves skin inflammation. The third active ingredient of the shampoo is keluamide (acetamide MEA or N-hydroxyethyl acetamine), has anti-inflammatory, antipruritic and keratolytic effect, it provides rapid elimination of scales from the skin surface.
The results of an open, non-comparative clinical study of the “Kelual DS” shampoo showed its high therapeutic efficacy in patients with seborrheic dermatitis of the scalp. Out of 20 patients who received a shampoo course for 4 weeks, clinical recovery was observed in 13 people (65%), a significant improvement was in 7 (35%) [55]. The shampoo was applied every other day, the exposure time on the head was 3 minutes. It is important to observe the duration of exposure, taking into account the fungicidal action of ciclopirox on Malassezia fungi.
Seborrheic dermatitis is a recurrent skin disease, therefore the cosmetic properties of the shampoo, its cleansing and conditioning properties are especially important for users. A survey of patients using Kelual DS shampoo for therapeutic purposes showed that the shampoo meets the highest cosmetic requirements: it has a rich creamy texture, forms a thick foam on the hair, is hypoallergenic, has a pleasant odour, gives shine to hair and does not make it difficult to comb [55].
Modern approaches to treatment
Genomic technologies have allowed us to better understand the nature of dandruff and seborrheic dermatitis, accurately diagnose the pathogen, and more effectively treat the disease. Effective antifungal agents are available in the formulas of cosmetic products, which not only treat the scalp, but also leave the hair shiny and easy to style. Modern formulas of shampoos and conditioners allow active therapeutic substances to remain on the hair and skin after rinsing them with water. Clinical studies show that patients with dandruff and seborrheic dermatitis more often use products that are pleasant to use than medical products. Preventive treatment can relieve dandruff and seborrheic dermatitis for a long time. Studies conducted over several years show that long-term prophylaxis prevents recurrence of the disease and M. globosa does not acquire resistance to antifungal agents, in particular to zinc pyrithione [13]. Some formulas of shampoos and lotions containing active anti-dandruff ingredients are quite soft and suitable for daily use. In particular, the use of lotion with active zinc “Kelual Zinc” allows you to fight dandruff at any time of the day: its use is not associated with washing the head, as the drug is applied to dry hair (but it is possible to wet, after washing). Advances in the production technology of medicinal and cosmetic products, including hygiene products, improved treatment methods provide better control of hair condition, fight dandruff and eliminate scalp discomfort, which undoubtedly leads to improved quality of life for patients with seborrheic dermatitis.
Literature references
- Суворова К.Н., Сысоева Т.А. Десквамативные поражения волосистой части головы. Учебное пособие. М., 2005.
- J Gray. Dandruff. Aetiology, pathophysiology and treatment. Blacwell, 2003.
- Schuster S. The aetiology of dandruff and the mode of action of therapeutic agents. Br J Dermatol 1984; 111: 235–42.
- Crespo Erchiga V, Ojega Martos A, Vera Casano A et al. Malassezia globosa as the causative agent of Pityriasis versicolor. Br J Dermatol 2000; 143: 799–803.
- Gueho E, Midgley G, Guillot J. The Genus Malassezia with description of four new species. Antonie Van Leeuwenhoek 1996; 69: 337–55.
- Boekhout T, Kamp M, Gueho E. Molecular typing of Malassezia species with PFGE and RPD. Med Mycol 1998; 36: 365–72.
- Makimura K, Tamura Y, Kudo M et al. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Med Microbiol 2000; 49: 29–35.
- Mayser P, Haze P, Papavassilis C et al. Differentation of Malassezia species: selectivity of cremophor EL, castor oil and ricinoleic acid for M.furfur. Br J Dermatol 1997; 137: 208–13. 9. Mittag H. Mycoses. 1995; 38: 13–21.
- Brasch J, Martens H, Sterry W. Arch Dermatol 1973; 107: 392–4.
- Kiffer M. J Amer Dermatol 1990; 22: 739–42.
- Nishimura K, Asada J, Tanaka S, Watanabe S. J Med Vet Mycol 1991; 29 (6): 387–93.
- Gemmer CM, DeAngelis YM, Theelan B et al. Fast, no-invasive method for molecular detection and speciation of Malassezia on human skin and application to dandruff microbiology. J Clin Microbiol 2002; 40: 3350–7.
- Boekhout T, Theelen B. Molecular Epidemiology of Malassezia Yeast (n.p.) 2001; 18: S332.
- Dawson TL Jr, DeAngelis YM, Treadway R et al. Dandruff. Part II: Dandruff and seborrheic dermatitis result from oleic acid released from sebum by Malassezia lipase activity. Poster present at Am Acad Dermatol Meeting, 2002.
- Gupta AK, DeAngelis YM, Kaczvinsky JR et al. Application of novel molecular methods to delineate the role of specific Malassezia species in the etiology of dandruff. Poster present at Am Acad Dermatol Meeting, 2002.
- Crespo Erchiga V, Ojega Martos A, Vera Casano A et al. Malassezia globosa as the causative agent of Pityriasis versicolor. Br J Dermatol 2000; 143: 799–803.
- Farthing CF, Staughton RC, Rowland Payne CM. Skin disease in homosexual patients with acquired immune deficiency syndrome (AIDS) and lesser forms of human T cell leukaemia virus (HTLV III) disease. Clin Exp Dermatol 1985; 10: 3–12.
- Smith KJ, Skelton HG, Yeager J et al. Cutaneous findings in HIV-i-positive patients: a 42-month prospective study. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). J Am Acad Dermatol 1994; 31: 746–54.
- Binder RL, Jonelis FJ. Seborrheic dermatitis in neuroleptic-induced parkinsonism. Arch Dermatol 1983; 119: 473–5.
- Faergemann J, Frederiksson T. Tinea versicolor with regard to seborrheic dermatitis. An epidemiological investigation. Arch Dermatol 1979; 115: 966–8.
- Maietta G, Fornaro P, Rongioletti F et al. Patients with mood depression have a high prevalence of seborrhoeic dermatitis. Acta Derm Venereol 1990; 70: 432–4.
- Tegner E. Seborhoeic dermatitis of the face induced by PUVA treatment. Acta Derm Venereol 1983; 63: 335–9.
- Bergbrant IM, Johansson S, Robbins D et al. An immunological study in patients with seborrhoeic dermatitis. Clin Exp Dermatol 1991; 16: 331–8.
- Midgley G, Hay RJ. Serological responses to Pityrosporum (Malassezia) in seborrhoeic dermatitis demonstrated by ELISA and western blotting. Bull Soc Fr Mycol Med 1988; 17: 267–76.
- Parry ME, Sharpe GR. Seborrhoeic dermatitis is not caused by an altered immune response to Malassezia yeast. Br J Dermatol 1998; 139: 254–63.
- Faergemann J, Bergbrant IM, Dohse M et al. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol 2001; 144: 549–56.
- Gupta AK, Bluhm R, Cooper EA et al. Seborrheic dermatitis. Dermatol Clin 2003; 21: 401–12.
- Pierard GE. Seborrheic dermatitis today, gone tomorrow? The link between the biocene and treatment. Dermatology 2003; 206: 187–8.
- Danby FW, Maddin WS, Margesson LJ et al. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2,5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol 1993; 29: 1008–12.
- Ford GP, Farr PM, Ive FA et al. The response of seborrhoeic dermatitis to ketoconazole. Br J Dermatol 1984; 111: 603–7.
- Meshkinpour A, Sun J, Weinstein G. An open pilot study using ointment in the treatment of seborrheic dermatitis. J Am Acad Dermatol 2003; 49: 145–7.
- Scaparro E, Quadri G, Virno G et al. Evaluation of the efficacy and tolerability of oral terbinafine (Daskil) in patients with seborrhoeic dermatitis. A multicentre, randomized, investigator-blinded, placebo-controlled trial. Br J Dermatol 2001; 144: 854–7.
- Vardy DA, Zvulunov A, Tchetov T et al. A double-blind, placebo-controlled trial of a ciclopirix olamine 1% shampoo for the treatment of scalp seborrheic dermatitis. J Dermatol Treat 2000; 11: 73–7.
- Marks R, Pearse AD, Walker AP. The effects of a shampoo containing oyrithione zinc on the control of dandruff. Brit J Dermatol, 1985; 112: 415–22.
- ДмитриевГ.А., CамсоновВ.А., НаволоцкаяТ.И., БрагинаЕ.Е. Оценка действия шампуня, содержащего пиритион цинка, против Pityrosporum ovale, участвующего в возникновении перхоти. Вестн. дерматол. и венерол. 1999; 5: 57–9. 37. Widal. 2002; с. Б-412.
- Korting HC, Grundmann-Kollmann M. The hydroxypyridones: a class of antimycotics of its own. Mycoses 1997; 40: 243–7.
- del Palacio-Hernanz A, Guarro-Artigas J, Figueras-Salvat MJ et al. Changes in fungal ultrastructure after short-course ciclopiroxolamine therapy in Pityriasis versicolor. Clin Exp Dermatol 1990; 15: 95–100.
- Abrams BB, Hanel H, Hoehler T. Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol 1991; 9: 471–7.
- Gupta AK, Skinner AR. Ciclopirox for the treatment of superficial fungal infections: a review. Int Dermatol 2003; 42 (Suppl. 1): 3–9.
- Gupta AK. Ciclopirox: an overview. Int J Dermatol 2001; 40: 305–10.
- Hanel H, Smith-Kurtz E, Pastowsky S. [Therapy of seborrheic eczema with an antifungal agent with an antiphlogistic effect]. Mycoses 1991; 34 (Suppl. 1): 91–3. 44. Widal. 1996; с. Б-87.
- Gray J. Dandruff. Aetiology, pathophysiology and treatment. Blacwell, 2003.
- Altmeyer P, Plott T. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double blind, vehicle-controlled trial. Int J Dermatol 2004.
- Abeck D. Rationale of frequency of use of ciclopirox 1% shampoo in the treatment of seborrheic dermatitis: results of a double-blind, placebo-controlled study comparing the efficacy of once, twice, and three times weekly usage. Int J Dermatol 2004.
- Lebwohl M. Safety and efficacy of ciclopirox 1% shampoo for the treatment of seborrheic dermatitis of the scalp in the US population: results of a double-blind, vehicle-controlled trial. Int J Dermatol 2004.
- Dupuy P, Maurette C, Amoric JC, et al. Randomized, placebo-controlled, double-blind study on clinical efficacy of ciclopiroxolamine 1% cream in facial seborrheic dermatitis. Br J Dermatol 2001; 144: 1033–7.
- Unholzer A, Varigos G, Nicholls D et al. Ciclopiroxolamine cream for treating seborrheic dermatitis: a double- blind parallel group comparison. Infection 2002; 30: 373–6.
- Aly R, Katz HI, Kempers SE et al. Ciclopirox gel for seborrheic dermatitis of the scalp. Int J Dermatol 2003; 42 (Suppl. 1): 19–22.
- Shuttleworth D, Squire RA, Boorman GC et al. Comparative clinical efficacy of shampoos containing ciclopirox olamine (1,5%) or ketoconazole (2%, Nizoral) for the control of dandruff/seborrheic dermatitis. J Dermatol Treat 1998; 9: 157–62.
- Squire RA, Goode K. A randomized, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1,5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral) for the treatment of dandruff/seborrhoeic dermatitis. J Dermatol Treat 2002; 13: 51–60.
- Gupta AK, Nicol KA. Ciclopirox 1% shampoo for the treatment of seborrheic dermatitis. Internat J Dermatol 2006; 45 (1): 66–9.
- Гаджигороева А.Г. Новые возможности в лечении себорейного дерматита волосистой части головы. Клин. дерматол. и венерол. 2005; 2: 70–2.
Seborrheic dermatitis has the same aetiology as dandruff, but with much more pronounced clinical manifestations. Often, inflamed skin is very itchy and oozing. Flakes can be white or yellow. Peeling extends beyond the scalp to hairy or bare skin. Seborrheic dermatitis is a chronic infectious disease of the skin surface, which forms in the foci of the body rich in sebaceous glands, especially in the area of the nasolabial triangle and scalp. The disease occurs in individuals of both sexes, but more in men. The tendency to activate the pathological process is observed in the dry frosty months, in contrast to the summer, when the clinical manifestations of the disease may be absent.















 Back
Back

